Technology Maturation Awards

Technology Maturation Awards (TMAs) are designed to help promising but yet unlicensed technologies attract external partners. TMAs support efforts to demonstrate the commercial potential of science and engineering innovations through proof-of-concept, prototyping, technology development and/or scale-up work. Successful TMA projects generate the validation necessary to attract experienced licensees, and on average generate a 10x return on investment on future grants.
Guidelines:
- Type of award: Grant or continuing grant
- Estimated number of awards: The Technology Commercialization Office (TCO) may fund two (2) awards this round. Projects may receive up to $50,000 for a term of 6–12 months per award.
- Eligibility: Proposals may be submitted by GW faculty inventors with a GW-owned invention available for licensing (optioned inventions are eligible). Applicants with a new idea must disclose it to TCO before submitting a TMA proposal.
- Limit on number of proposals per PI or Co-PI: Two (2)
- Deadline: October, 19 2025.
- Applicants that have completed a two-week Lean Startup Short Course for the technology with the Office of Innovation & Entrepreneurship will be preferred. Please reference the course and learnings in your proposal.
From Innovation to Market: Success Stories
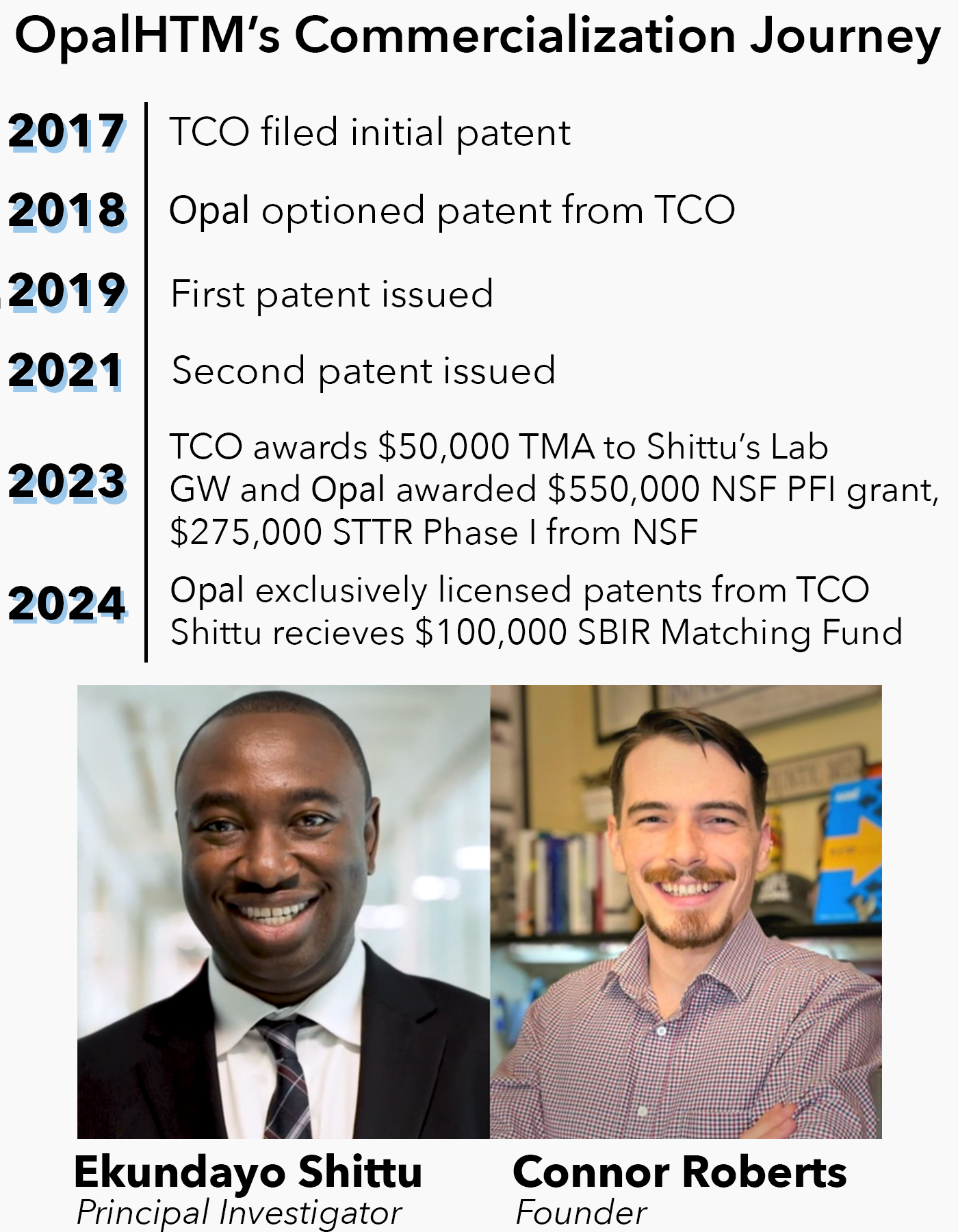
After receiving a Technology Maturation Award (TMA) in 2023, Dr. Ekundayo Shittu secured an NSF grant to advance his research. Shittu's student Connor Roberts then launched OpalHTM, going on to earn a Phase I STTR award. Supported by a GW subaward, a formal patent license, and continued development, OpalHTM became the first recipient of TCO’s $100,000 SBIR Matching Fund. Today, the company is completing the buildout of its products that will soon support critical healthcare infrastructure.
“I’m grateful to the GW TCO for fueling our next stage of development through their trailblazing SBIR Matching Fund. This program gives GW licensees like Opal HTM a massive leg up.”
— Connor Roberts, Founder of Opal HTM

